Here's yet one more device company that is offering a simplified monitoring system and below is a couple paragraphs from the website so again this looks like we have “data for sale’ here once again.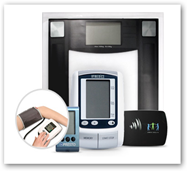 It is using a web portal and requires a USB connection to your computer. Controlling diabetes and hypertension are the main chronic conditions targeted with the system and the targeted sales for the service/devices are insurance companies and home healthcare agencies.
It is using a web portal and requires a USB connection to your computer. Controlling diabetes and hypertension are the main chronic conditions targeted with the system and the targeted sales for the service/devices are insurance companies and home healthcare agencies.
The company also plans to move into the “live sensor” area next as well and currently will send you text messages and reminders. Again the privacy policy opens up the information for aggregated information profiles and there’s the usual mumbo jumbo about how they use it, etc.
“Permission is required before AH discloses Personal Information to a third party. No permission is necessary for Aggregate Information, since Aggregate Information does not identify a specific individual.
a. For disclosure of Medical Information, Informed Consent is required before AH discloses Medical Information to a third party. Informed Consent occurs when an individual has sufficient facts about the disclosure, comprehends those facts, and voluntarily consents to the disclosure. Where a third party such as the employer or healthcare provider of an individual requires the individual to participate in an AH program which collects Medical Information, AH will require the employer or healthcare provider to procure Informed Consent before AH will release Medical Information to that employer or healthcare provider.”
There are a few devices out there that do not sell your data and the Tri-Coder is one under development. Again I wonder how many of these mobile device companies and apps can stay in business without selling data at times. In the article it also states such information could be available to employers and most folks I have been talking with of late are telling me they prefer to stay outside the radar and have tried some of the wellness programs but some have become way too intrusive with time and reporting so it will be interesting as consumers become more aware of the data selling epidemic as to how many folks actually will use the various technologies. BD
Data Floating Around the Web and You Don’t Know How It Got There? Time to License and Excise Tax Data Sellers–Identify “Flawed Data” Epidemic At The Root of the Problem
Aging in place and disease management technology developer Ambio Health has gained FDA 510(k) regulatory clearance for its flagship Ambio Remote Health Monitoring System. The system, first introduced at the Consumer Electronics Show in January, essentially adds wireless capability to standard home health monitors and automates data collection.
Stamford, Connecticut-based Ambio is now free to sell the system in the US, and it will do so at a price point significantly lower than competitors, according to CEO Kevin Jones. The wireless gateway, which plugs into a standard home-based Ethernet router, costs $19.99 by itself or $44.97 bundled with an AgaMatrix Presto blood-glucose monitor and proprietary Ambio wireless connector. A package with a Homedics BPA-060 blood-pressure monitor is priced at $89.97.
An Ambio Health-branded digital weight scale, sold for $84.98 with a wireless gateway or $64.99 without, has the transmitter built in. Monitoring service costs $4.99 a month per device, based on an annual subscription.
http://mobihealthnews.com/24345/fda-clears-ambio-health-wireless-health-monitoring-system/
No comments:
Post a Comment